If you’ve ever looked at a chemical equation and wondered why it has to be balanced, you’re not alone. This is one of those chemistry topics that feels confusing at first, but once it clicks, it actually makes sense.
In this post, I’ll explain balancing chemical equations in a simple, step-by-step way, using examples that students usually see in school exams.
What Is a Chemical Equation?
A chemical equation is just a way of showing a chemical reaction using symbols.
It tells us two basic things:
- what substances are reacting
- what new substances are formed
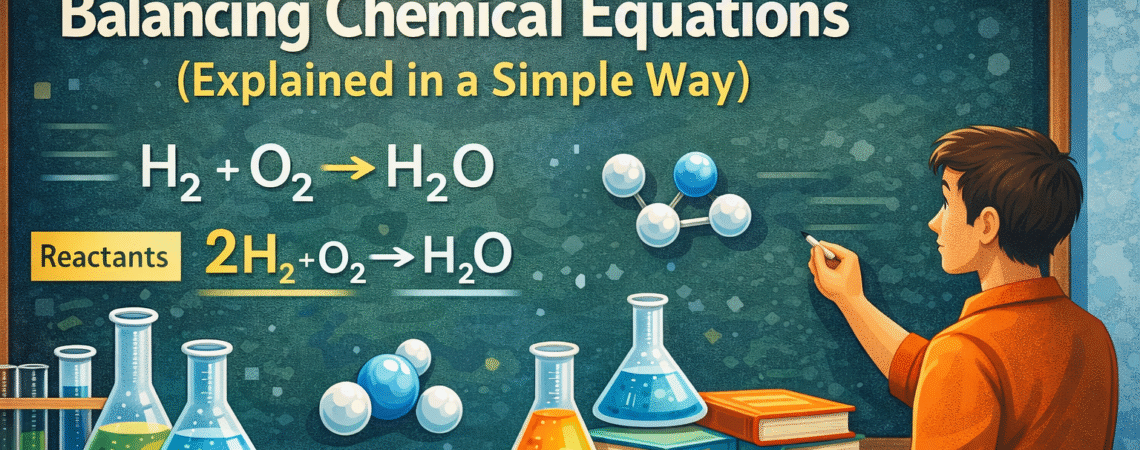
For example:
H₂ + O₂ → H₂O
This shows hydrogen reacting with oxygen to form water. Nothing fancy yet.
What Does “Balancing Chemical Equations” Mean in Chemistry?
When we say an equation is balanced, we mean the number of atoms of each element is the same on both sides.
Why does this matter?
Because atoms don’t disappear during a reaction. They only rearrange.
If you start with 2 oxygen atoms, you must end with 2 oxygen atoms. Chemistry doesn’t allow shortcuts.
Why Do We Need to Balance Chemical Equations?
Students often ask this, especially during exams.
Balancing is important because:
- it follows the law of conservation of mass
- it shows correct proportions of reactants
- it helps in numerical problems
- unbalanced equations are considered incorrect
In short, an unbalanced equation does not represent a real reaction.
Balancing Chemical Equations (Step by Step)
There isn’t one single “magic formula” that works instantly. But there is a logical approach that works most of the time. You can use this tool.
Chemical Equation Balancer
Enter an unbalanced chemical equation (example: H2 + O2 = H2O)
Note: This chemical equation balancer is designed for basic educational use and common school-level reactions.
Oxidation Number Calculator
Learn how to calculate oxidation numbers step by step with clear explanations.
Learn More →Mole Concept Explained
Understand the mole concept with simple examples commonly used in exams.
Read Guide →Chemistry Calculators
Explore helpful chemistry calculators for students and exam preparation.
View Tools →Let’s walk through it.
Step 1: Write the Unbalanced Equation Clearly
Always start with the correct chemical formulas.
Example:
Fe + H₂O → Fe₃O₄ + H₂
Don’t rush this step. A small formula mistake causes big confusion later.
Step 2: Count Atoms on Both Sides
Now, count each element separately.
- Iron (Fe): left side = 1, right side = 3
- Oxygen (O): left side = 1, right side = 4
- Hydrogen (H): left side = 2, right side = 2
Clearly, this is not balanced yet.
Step 3: Balance One Element at a Time
Most teachers suggest starting with metals first.
So we balance iron by placing a coefficient in front of Fe:
3Fe + H₂O → Fe₃O₄ + H₂
Notice something important here — we did not change Fe₃O₄. We only added a number in front.
Step 4: Adjust the Remaining Elements
Now oxygen and hydrogen are still unbalanced.
After adjusting coefficients carefully, the balanced equation becomes:
3Fe + 4H₂O → Fe₃O₄ + 4H₂
Step 5: Do a Final Check (Very Important)
Count atoms again on both sides.
If everything matches, your equation is balanced.
Skipping this step is a common exam mistake.
Examples of Balanced Chemical Equations
Let’s look at a few common ones students often practice.
Example 1: Formation of Water
Unbalanced:
H₂ + O₂ → H₂O
Balanced:
2H₂ + O₂ → 2H₂O
Example 2: Aluminum and Oxygen
Unbalanced:
Al + O₂ → Al₂O₃
Balanced:
4Al + 3O₂ → 2Al₂O₃
Example 3: Combustion Reaction
Unbalanced:
C₃H₈ + O₂ → CO₂ + H₂O
Balanced:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
Common Mistakes Students Make
These are very common, especially in exams:
- changing subscripts instead of coefficients
- balancing hydrogen or oxygen too early
- forgetting atoms inside brackets
- not rechecking the final equation
Most mistakes happen due to rushing.
A Few Practice Questions
Try these without looking at the answers:
Mg + HCl → MgCl₂ + H₂
Na + H₂O → NaOH + H₂
Zn + HNO₃ → Zn(NO₃)₂ + H₂
Practicing regularly improves confidence.
Final Thoughts
Balancing chemical equations is not about memorizing rules. It’s about understanding that atoms must be conserved.
Once you slow down, count carefully, and adjust coefficients step by step, the process becomes much easier.
This is one topic where practice truly makes perfect.
